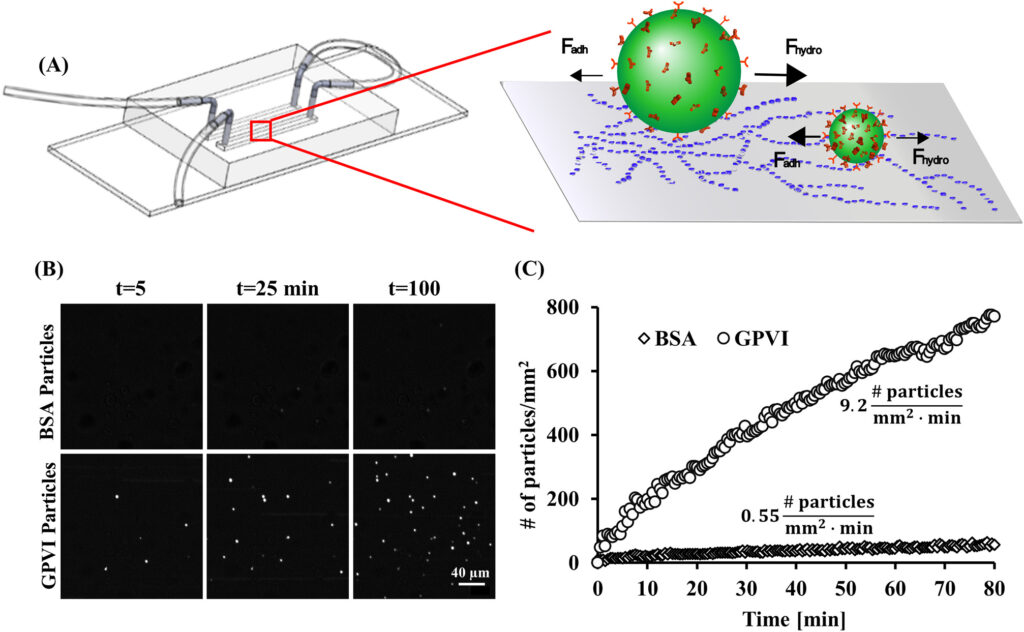
 Glycoprotein VI (GPVI)-functionalized nanoparticles targeting arterial injury sites under physiological flow (2020)
Glycoprotein VI (GPVI)-functionalized nanoparticles targeting arterial injury sites under physiological flow (2020)
Moran Levi, Mark Epshtein ,Tatsiana Castor, Meinrad Gawaz, Netanel Korin
Thrombus formation at athero-thrombotic sites is initiated by the exposure of collagen followed by platelet adhesion mediated by the platelet-specific collagen receptor glycoprotein VI (GPVI). Here, dimeric GPVI was used as a targeting motif to functionalize polymeric nanoparticle-based drug carriers and to show that with proper design, such GPVI-coated nanoparticles (GPNs) can efficiently and specifically target arterial injury sites while withstanding physiological flow. In a microfluidic model, under physiological shear levels (1-40 dyne/cm2), 200 nm and 2 μm GPNs exhibited a >60 and >10-fold increase in binding to collagen compared to control particles, respectively. In vitro experiments in an arterial stenosis injury model, subjected to physiological pulsatile flow, showed shear-enhanced adhesion of 200 nm GPNs at the stenosis region which was confirmed in vivo in a mice ligation carotid injury model using intravital microscopy. Altogether, our results illustrate how engineering tools can be harnessed to design nano-carriers that efficiently target cardiovascular disease sites.
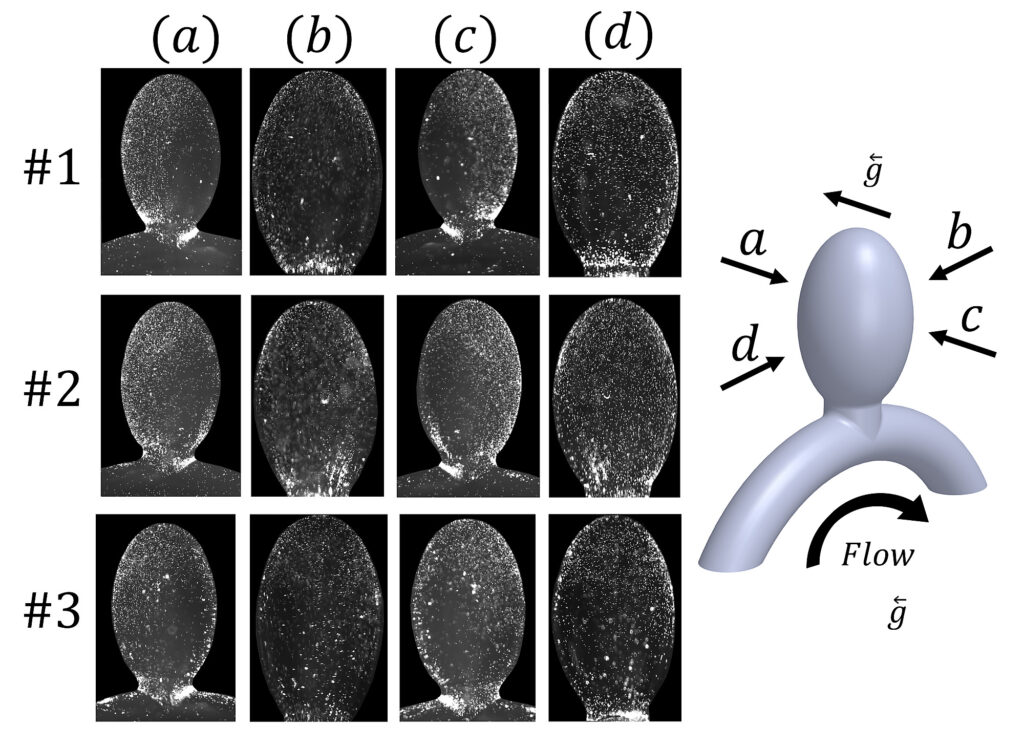
Computational and experimental investigation of particulate matter deposition in cerebral side aneurysms (2020)
Mark Epshtein and Netanel Korin
Intracranial aneurysms frequently develop blood clots, plaque and inflammations, which are linked to enhanced particulate mass deposition. In this work, we propose a computational model for particulate deposition, that accounts for the influence of field forces, such as gravity and electrostatics, which produce an additional flux of particles perpendicular to the fluid motion and towards the wall. This field-mediated flux can significantly enhance particle deposition in low-shear environments, such as in aneurysm cavities. Experimental investigation of particle deposition patterns in in vitro models of side aneurysms, demonstrated the ability of the model to predict enhanced particle adhesion at these sites. Our results showed a significant influence of gravity and electrostatic forces (greater than 10%), indicating that the additional terms presented in our models may be necessary for modelling a wide range of physiological flow conditions and not only for ultra-low shear regions. Spatial differences between the computational model and the experimental results suggested that additional transport and fluidic mechanisms affect the deposition pattern within aneurysms. Taken together, the presented findings may enhance our understanding of pathological deposition processes at cardiovascular disease sites, and facilitate rational design and optimization of cardiovascular particulate drug carriers.
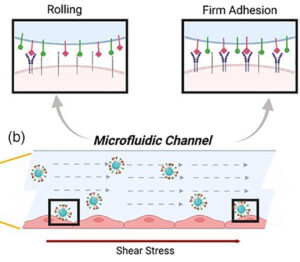
Targeting functionalized nanoparticles to activated endothelial cells under high wall shear stress (2019)
Hila Zukerman, Maria Khoury, Yosi Shammay, Josué Sznitman, Noah Lotan, Netanel Korin
Local inflammation of the endothelium is associated with a plethora of cardiovascular diseases. Vascular‐targeted carriers (VTCs) have been advocated to provide focal effective therapeutics to these disease sites. Here, we examine the design of functionalized nanoparticles (NPs) as VTCs that can specifically localize at an inflamed vessel wall under pathological levels of high shear stress, associated for example with clinical (or in vivo) conditions of vascular narrowing and arteriogenesis.
To test this, carboxylated fluorescent 200 nm polystyrene particles were functionalized with ligands to activated endothelium, that is, an E‐selectin binding peptide (Esbp), an anti ICAM‐1 antibody, or using a combination of both. The functionalized NPs were investigated in vitro using microfluidic models lined with inflamed (TNF‐α stimulated) and control endothelial cells (EC). Specifically, their adhesion was monitored under different relevant wall shear stresses (i.e., 40–300 dyne/cm2) via real‐time confocal microscopy. Experiments reveal a significantly higher specific adhesion of the examined functionalized NPs to activated EC for the window of examined wall shear stresses. Moreover, particle adhesion correlated with the surface coating density whereby under high surface coating (i.e., ~10,000 molecule/particle), shear‐dependent particle adhesion increased significantly. Altogether, our results show that functionalized NPs can be designed to target inflamed endothelial cells under high shear stress. Such VTCs underscore the potential for attractive avenues in targeting drugs to vasoconstriction and arteriogenesis sites.
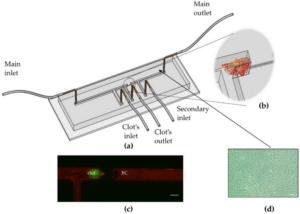 Endothelial Cell Activation in an Embolic Ischemia-Reperfusion Injury Microfluidic Model (2019)
Endothelial Cell Activation in an Embolic Ischemia-Reperfusion Injury Microfluidic Model (2019)
Danielle Nemcovsky Amar, Mark Epshtein, Netanel Korin
Ischemia, lack of blood supply, is associated with a variety of life-threatening cardiovascular diseases, including acute ischemic stroke and myocardial infraction. While blood flow restoration is critical to prevent further damage, paradoxically, rapid reperfusion can increase tissue damage. A variety of animal models have been developed to investigate ischemia/reperfusion injury (IRI), however they do not fully recapitulate human physiology of IRI. Here, we present a microfluidic IRI model utilizing a vascular compartment comprising human endothelial cells, which can be obstructed via a human blood clot and then re-perfused via thrombolytic treatment. Using our model, a significant increase in the expression of the endothelial cell inflammatory surface receptors E-selectin and I-CAM1 was observed in response to embolic occlusion. Following the demonstration of clot lysis and reperfusion via treatment using a thrombolytic agent, a significant decrease in the number of adherent endothelial cells and an increase in I-CAM1 levels compared to embolic occluded models, where reperfusion was not established, was observed. Altogether, the presented model can be applied to allow better understanding of human embolic based IRI and potentially serve as a platform for the development of improved and new therapeutic approaches.
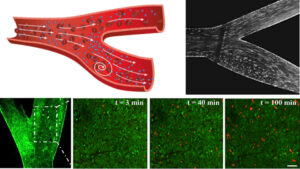 Mapping deposition of particles in reconstructed models of human arteries (2019)
Mapping deposition of particles in reconstructed models of human arteries (2019)
Maria Khoury, Mark Epshtein, Hikaia Zidan, Hila Zukerman, Netanel Korin
Targeted drug delivery to diseased vasculature, such as atherosclerotic lesions, is a multistep process, which is based on the transport of drug carriers to a selected region and their deposition at the desired destination. Current modeling approaches, including microfluidics and animal models, fail to accurately simulate this multi-scale process in human arteries, where blood flow is dominant. Here we study particle deposition in endothelialized 3D reconstructed models of the human carotid bifurcation under physiological hemodyamic conditions. Our results showed that particle localization is highly dependent on vessel geometry and local flow features. Additionally, while strongly adhesive particles tend to adhere more profoundly at high-shear regions, associated with athero-thrombosis, enhanced deposition at vascular flow regions, associated with inflammation and plaque accumulation, e.g., recirculation flows, can be achieved using weakly adhesive particles. Moreover, pulsatile flow as well as presence of blood cells significantly reduce particle adhesion and affect their deposition pattern. These findings highlight the key role of vessel geometry, hemodynamics and particle characteristics in the optimizing vascular targeting nano-carriers.
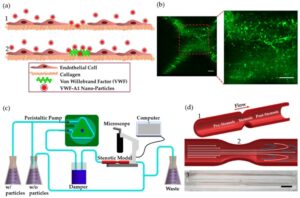 The Flow Dependent Adhesion of von Willebrand Factor (VWF)-A1 Functionalized Nanoparticles in an in Vitro Coronary Stenosis Model (2019)
The Flow Dependent Adhesion of von Willebrand Factor (VWF)-A1 Functionalized Nanoparticles in an in Vitro Coronary Stenosis Model (2019)
Yathreb Asaad, Mark Epshtein, Andrew Yee, Netanel Korin
In arterial thrombosis, von Willebrand factor (VWF) bridges platelets to sites of vascular injury. The adhesive properties of VWF are controlled by its different domains, which may be engineered into ligands for targeting nanoparticles to vascular injuries. Here, we functionalized 200 nm polystyrene nanoparticles with the VWF-A1 domain and studied their spatial adhesion to collagen or collagen-VWF coated, real-sized coronary stenosis models under physiological flow.
When VWF-A1 nano-particles (A1-NPs) were perfused through a 75% stenosis model coated with collagen-VWF, the particles preferentially adhered at the post stenotic region relative to the pre-stenosis region while much less adhesion was detected at the stenosis neck (~ 65-fold less). When infused through collagen-coated models or when the A1 coating density of nanoparticles was reduced by 100-fold, the enhanced adhesion at the post-stenotic site was abolished. In a 60% stenosis model, the adhesion of A1-NPs to collagen-VWF-coated models depended on the location examined within the stenosis. Altogether, our results indicate that VWF-A1 NPs exhibit a flow-structure dependent adhesion to VWF and illustrate the important role of studying cardiovascular nano-medicines in settings that closely model the size, geometry, and hemodynamics of pathological environments.
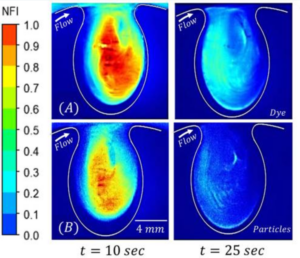
Mapping the Transport Kinetics of Molecules and Particles in Idealized Intracranial Side Aneurysms (2018)
Mark Epshtein and Netanel Korin
Intracranial side aneurysms (IA) are pathological blood-filled bulges in cerebral blood vessels. Unlike healthy blood vessels where mass transport is dominated by convection, both diffusion and convection can play an active role in aneurysm sites. Here, we study via dye washout experiments and numerical simulations, the transport characteristics of particles (1 micron) and small molecules (300 Da) into simplified side aneurysms models following bolus injection.
Time-lapse fluorescent microscopy imaging performed in our idealized aneurysm models showed that the parent artery geometry (located on the inner vs. outer curvature) as well as the aneurysm aspect ratio (AR) affect the washout kinetics while the pulsatile nature of the flow, maintained within the physiological range, carries only a minor effect. Importantly, in the absence of effective diffusion, particles that are located on slow streamlines linger within the aneurysm cavity, a phenomenon that could be of importance in deposition of cells and nano/micro-particles within aneurysms. Altogether, mass transport studies may provide valuable insights for better understanding of aneurysm pathophysiology as well as for the design of new diagnostic and theranostic nano-medicines.
 Glycoprotein VI (GPVI)-functionalized nanoparticles targeting arterial injury sites under physiological flow (2020)
Glycoprotein VI (GPVI)-functionalized nanoparticles targeting arterial injury sites under physiological flow (2020)

 Endothelial Cell Activation in an Embolic Ischemia-Reperfusion Injury Microfluidic Model (2019)
Endothelial Cell Activation in an Embolic Ischemia-Reperfusion Injury Microfluidic Model (2019) Mapping deposition of particles in reconstructed models of human arteries (2019)
Mapping deposition of particles in reconstructed models of human arteries (2019) The Flow Dependent Adhesion of von Willebrand Factor (VWF)-A1 Functionalized Nanoparticles in an in Vitro Coronary Stenosis Model (2019)
The Flow Dependent Adhesion of von Willebrand Factor (VWF)-A1 Functionalized Nanoparticles in an in Vitro Coronary Stenosis Model (2019)