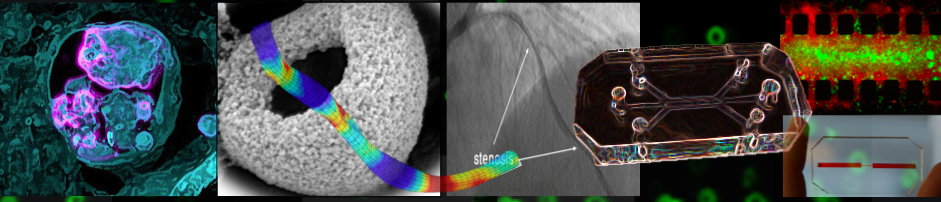
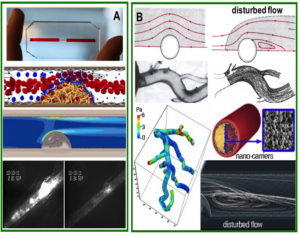
Our Research is focused on studying the interplay between hemodynamics, vascular physiology, and transport phenomena in vascular diseases. The long-term objective of this research is to allow better understanding of the biophysical determinants of vascular disease and to leverage this knowledge to develop innovative therapeutic approaches. This interdisciplinary research is preformed through integrating principles of fluid dynamics, tissue biomechanics, vascular physiology and biomaterials science.
Overview:
Pathological Hemodynamics
Hemodynamic parameters play a major role in the circulatory system and are tightly regulated under normal physiological conditions. Abnormal features of local blood flow (e.g., low/high shear stress, eddies, etc) have been associated with a variety of vascular diseases including: atherosclerosis, aneurisms, stroke and vaso-spasm. Unraveling the biophysical features of abnormal flow and their role in vascular disease may be valuable for understanding the etiology and natural history of these pathologies as well as optimizing current therapies and developing new therapeutic strategies.We are particularly interest in leveraging local biophysical abnormalities as mechanisms to target nano-medicine to sites of disease. For example, we have previously utilized abnormal elevated shear stress at stenotic sites as a mechanism to concentrate nano-carriers at these locations.
Vaso-Occlusion in Erythrocyte Mechanical Disorders
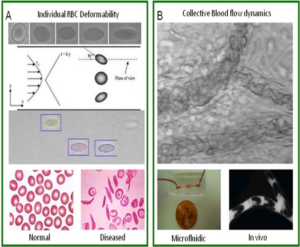 Various pathophysiological conditions including sickle cell disease, diabetes, peripheral vascular diseases and sepsis are associated with abnormalities in erythrocyte mechanics. As the mechanical properties of Red Blood Cells (RBCs) are primary determinants of blood behavior, abnormal RBCs mechanics affects blood flow dynamics in the circulation and is associated with a variety of pathological events including occlusion of flow. Current in vitro assays which focus on single cell mechanics, lack the ability to address the key pathological events occurring in vivo where collective behavior and dynamic biophysical interactions between cells play a major role. For example, in sickle cell anemia vascular occlusion is a multistep complex process which ultimately results in collective jamming of blood flow induced by abnormally stiff RBCs. In order to properly model and study occlusion events in RBC disorders, we are developing microfludic systems that mimic blood flow in micro-vascular networks and recapitulate the biophysical dynamics and pathological outcomes of abnormalities in RBC mechanics. Using this system we perform quantitative analysis of blood flow characteristics as well as explore the dynamics of occlusive events under defined conditions.
Various pathophysiological conditions including sickle cell disease, diabetes, peripheral vascular diseases and sepsis are associated with abnormalities in erythrocyte mechanics. As the mechanical properties of Red Blood Cells (RBCs) are primary determinants of blood behavior, abnormal RBCs mechanics affects blood flow dynamics in the circulation and is associated with a variety of pathological events including occlusion of flow. Current in vitro assays which focus on single cell mechanics, lack the ability to address the key pathological events occurring in vivo where collective behavior and dynamic biophysical interactions between cells play a major role. For example, in sickle cell anemia vascular occlusion is a multistep complex process which ultimately results in collective jamming of blood flow induced by abnormally stiff RBCs. In order to properly model and study occlusion events in RBC disorders, we are developing microfludic systems that mimic blood flow in micro-vascular networks and recapitulate the biophysical dynamics and pathological outcomes of abnormalities in RBC mechanics. Using this system we perform quantitative analysis of blood flow characteristics as well as explore the dynamics of occlusive events under defined conditions.